With Lab-on-a-Chip technology, testing becomes much faster and more efficient. Not only can these sensors replace reference laboratories, they can even communicate the results directly to a computer for validation and statistics. And, as our expert Manuel Bäuscher wants to emphasize, the Lab-on-a-Chip is a non-invasive option for in-vitro diagnostics with portable desktop systems.
Although used primarily in a medical context, the technology can also help make testing for environmental influences far more efficient, be it for water pollution or soil composition diagnostics. But it is not just the time saved that makes these mini laboratories the more efficient choice. Multiple sensor surfaces for different detection jobs can measure several biomarkers in parallel with a single sample, making the Lab-on-a-Chip economical to use on top of its other benefits.
The jury is still out on whether the quality of testing is as precise as in a big laboratory, since many factors are at play in that equation: Quantitative results are more difficult to generate with the bio-electrical principle than qualitative results. The implication is that it becomes harder to determine the concentration of the tested sample.
In the case of COVID-19, this could mean the difference between testing whether someone is infected or whether this person is infectious, or how many antibodies have formed after an infection or vaccination. The relevant biomarkers can be detected either by optical means or by electronic means by analyzing liquid samples like blood, saliva, or sweat. RealIZM met Manuel Bäuscher to speak about two very interesting projects with the Lab-on-a-Chip.
Manuel, what made you interested in working on the idea of a Lab-on-a-Chip?
Manuel Bäuscher: It is just a very exiting topic. We were already working on this before Corona hit, and now this new experience has made all of us even more aware of how important developments can be in this field. What is crucial is the ability to react quickly and adapt the system, so that it could perhaps even be used in a novel pandemic scenario.
There are currently two Lab-on-a-Chip projects at Fraunhofer IZM. Can you explain what they are about?
Manuel Bäuscher: Well, in the glucose sensing project, we are using what we call our 2D printers: We are creating sensor structures by printing conductive ink onto foil substrates, which are then biologically functionalized with various media, such as glucose oxidase. There is an enzymatic exchange reaction at the end when combined with glucose. This is read out with an electrochemical sensor, as is currently the case with most glucose measuring devices.
This sensor can work with the results of a previous project, in which we developed sensors that can measure the moisture in diapers, e.g. to make it easier for nursing/caretaking staff to monitor patients with incontinence issues. Applications include nursing homes, where bedridden patients can have their diapers changed more precisely when they need them. In the same way, the biological values of a diabetes patient could be monitored as well.
Which materials are you using for these sensors?
Manuel Bäuscher: We areusing silver nanoparticles. One could think that silver is expensive, but it has actually become the economical choice to use it. There are now quite a few different manufacturers of these silver nanoparticle inks, who are making and trading vast quantities of the product. Silver is used as a material for many different purposes, not just sensors. It is interesting, because silver chloride electrodes can also be used, mostly as a reference electrode for electro-chemical sensors. Working with this material simplifies many things, because there is no need to look for any additional material.
What do you think personally: When will these diapers you mentioned earlier be ready for purchase, for example in online pharmacies?
Manuel Bäuscher: It depends. I don’t know if this product is at all interesting for domestic use. It will probably be more attractive for hospitals or nursing homes. The whole communication interface could be connected, so that nursing staff in the day room could just get a signal on the screen. This would tell them where they need to intervene in some way, where they need to change a diaper. And it would increase efficiency in the nursing workflow, as they don’t have to go from bed to bed and check everything by hand.
That sounds like a real improvement in their work. Can we talk a bit more about the technical side of this? How does the communication work between the sensors in the diapers and the computer?
Manuel Bäuscher: There are various concepts, but the most sensible option would be to work with magnetic clips. We could install these on the waistband or similar in the form of a small communication module. This then passes on the signals to an access point either via Bluetooth or wifi.
There are already other approaches, for example optical ones, that can be used to measure blood glucose. What is special about your sensor, about your system?
Manuel Bäuscher: We based our work on these humidity sensors that we wanted to integrate into diapers. At Fraunhofer IZM, working in cooperation with HTW Berlin, research has already been done in this direction for urine as the medium in question. So we pursued a similar path. What interests us is to see how many sensors can be integrated into one of these diapers for monitoring the health status of patients. It’s not just about blood glucose levels, but also about possibly testing other biomarkers in urine. You can establish different capabilities on the sensor surface to detect different markers. Because we wanted to find out more about this, we didn’t get involved with any other methods in the first place. Also, the printing technique we propose is very inexpensive and scalable for mass production.
So the diapers, to stay with our example, would be disposable?
Manuel Bäuscher: Exactly, for hygiene reasons, of course. But the nice thing is that such a sensor can be integrated into the diaper production process and does not have to be added afterwards.
Which makes it easier to integrate them, I guess? Very interesting. But I’m also interested in your recently finished Graph-PoC project. Can you tell us more about that?
Manuel Bäuscher: Graph-PoC is the project I started with here at Fraunhofer IZM. It was about developing a sensor platform with a new material, graphene, or graphene oxide, with which we are able to electrically detect infectious diseases in blood. This primarily involved bacterial infections. However, we originally planned for detecting multiple infectious diseases, from bacterial to viral infections. Due to the Coronavirus outbreak, our focus was expanded to viral biomarkers.
At the moment, we are in the process of validating reproducible measurements with qualitative results, which enables us to give the crucial yes-or-no answer: Is there an infection, yes or no? Which is similar to the SARS-CoV-2 antigen rapid tests we all know. We are also trying to adapt this for concentration dependence, which is proving to be quite difficult due to the new materials involved. But we are very flexible with the functionalization of the sensor surface. We just produced new sensors, specifically multi-sensor chips, which means that we now have eight sensor areas. Each of them can be implemented with different capture molecules. Therefore, we could theoretically detect eight different things at once with one drop of blood.
Are these protein-based sensors?
Manuel Bäuscher: They are indeed. We employ a capture molecule on the sensor surface. That could be an antibody, for example. This antibody then catches the very specific biomarker in the sample, e.g. human blood. Another case could be that we have a viral envelope on our sensor, like parts of the SARS-CoV-2 virus. Then, we could catch and detect from antibodies in the blood sample whether someone already had COVID-19 or has been vaccinated against it.
Why did you choose graphene as your material if it still makes yes-no detection difficult? Are there no other materials you could have used for these sensors?
Manuel Bäuscher: That’s right. Graphene, particularly reduced graphene oxide, is a very novel material, which is currently on its way to market applications. The main application at the moment, from what I know, is a separator layer in the batteries of phones. Like silicon, it has insulating properties as graphene oxide or electrically conductive properties as graphene or reduced graphene oxide. And it’s very interesting for our case, because graphene is made of carbon. It is a bio-compatible material, which means that we can couple biomaterial to its functional chemical groups. With these functional groups and with our deposition technology, we can increase the sensor area in such a way that we can couple more biological material on the sensor surface than we could on a very flat, smooth, and plane gold surface. In fact, we place these flakes in such a way that a 3D arrangement is created and our sensor area increased.
And with this electrical approach, you can detect different diseases simultaneously?
Manuel Bäuscher: Well, as I said, that would be possible in principle. Antigen detection and antibody detection are based on the same principle. They are all protein-based systems that have different functional groups that we can couple to the surface of the sensor. What we wanted to achieve with the Graph-PoC project is a way to test up to three different biomarkers with a single blood sample. The aim was to detect bacterial infections. Basically, we want to detect blood poisoning at an early stage. There is a very specific biomarker in the blood for this, which we want to detect. Through the pandemic, actually from its very beginning, the consortium decided to also compile several target biomarkers in order to detect viral, bacterial, or even fungal infections simultaneously with this multisensory chip.
So the pandemic influenced the medium-term goal of the project. Did you already test this sensor system with real samples?
Manuel Bäuscher: We are currently functionalizing the sensor with a capture molecule and trying to detect a biomarker with artificial samples that we prepared. We know what concentration of this biomarker we are putting into the medium, and we are trying try to detect different concentrations and read out the electrical signal. However, we have not yet done this with blood, which is something we want to do later. We’re still missing too many measurements to be able to filter out noise or other proteins or maybe even cells that could be on the sensor surface. Measuring in blood is on a whole different level.
You already use the second generation of sensors. What is the difference between the first and the second generation? What did you improve?
Manuel Bäuscher: At the beginning, we developed different sensor designs, which we produced on wafer level. That means we produced different electrode designs and different sensor area diameters. Then we had a look at which design had the greatest signal differences. In terms of size, however, it didn’t quite fit, and we wasted space. For mass production technology, it’s essential to save space. Furthermore, we tested deposition techniques with the new material reduced graphene oxide on these sensors. We did that for each sensor individually, one by one. Of course, this is not economical. With this new second-generation sensor, we committed ourselves to a layout, i.e. to only one electrode geometry on the sensor surface. That’s why we can now coat all eight grades at once. This saves time. We are now trying to scale the whole thing up, so that perhaps even the entire wafer, on which we have 400 sensors, can be coated at once.
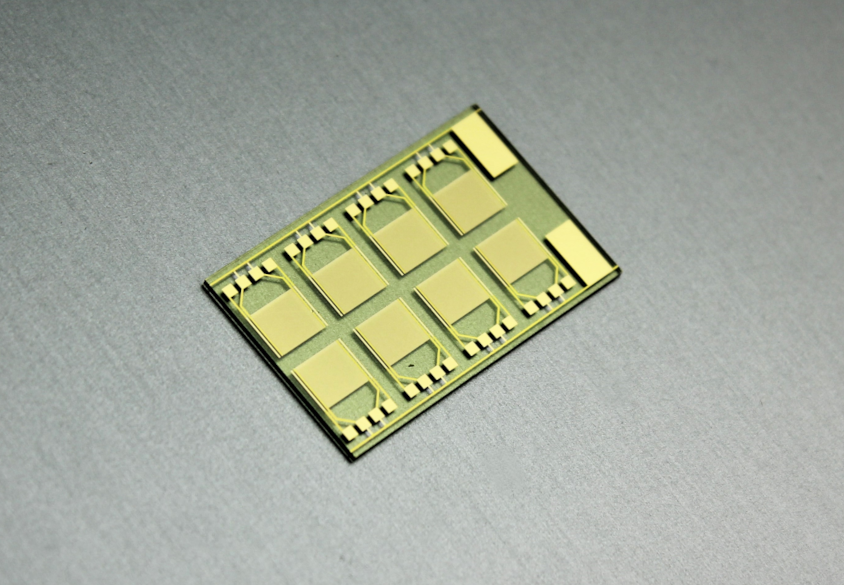
You are using reduced graphene for the sensors. But what kind of material did you use for the chips?
Manuel Bäuscher: For the chips, we are using a glass substrate. Because if we put a conductive material on silicon, such as these gold electrodes we use in Graph-PoC, we need a non-conductive material underneath, between the silicon and the metal, and that only works if we add an oxide combined with a silicon nitride to these purchased silicon wafers. Otherwise, currents would pass through the silicon from one electrode to the other. Even though glass is a bit more expensive to use at wafer level, we have no electrical influences through the substrate, because glass does not conduct anything and is completely insulated.
How long will it take until this sensor system reaches the market?
Manuel Bäuscher: We have been working on the system for three years now. I estimate that it will take just as long again to finish it up, probably even longer to bring it to the market. Unfortunately, it is not so trivial a task to gain the knowledge about all these biomolecules. I mean, I was personally very inexperienced at the beginning. I learned a lot from our project partners and from the colleagues at the Technical University’s department of Microbiology, who are part of the project. This topic is not easy. You have to evaluate functionalization and biomolecule coupling, because you cannot recognize these molecules under a microscope or even under a scanning electron microscope. That’s why we use light signals, like chemiluminescence to prove that the parts are indeed on our sensor surface. When and only when we are sure that they are binding specifically to the surface, we can carry out the electrical measurements.
What do you think, how expensive is this sensor system at the moment?
Manuel Bäuscher: In the Graph-PoC project, the microfluidics are produced afterwards, i.e. these sensors are embedded and microfluidics added later. This is done at the Technical University Berlin, more precisely by the Assembly and Encapsulation Technology group at Fraunhofer IZM / TUB. This is another process in which we try to make the system economically viable. We produce the sensors on wafers and try to carry out the deposition of reduced graphene oxide on wafer level, too. Afterwards, the chips are separated and embedded in a mold compound on wafer level or even on panel level, and a microfluidic is generated with a thick polymer. This makes the chip a bit more expensive than it is now. Our goal was to design the chip to costs less than €15. It was important that we can remain competitive with other systems.
Let’s talk about sustainability. When working on the chips, did you consider the question of recycling?
Manuel Bäuscher: That would be interesting. But for hygiene reasons, it’s not possible yet. Since we’re working with blood, the cleaning would be rather complicated. The biofunctionalization would probably also be destroyed, because these molecules would perhaps not be able to withstand isopropanol or acetone. But in general, a regeneration of these molecules would be possible.
Who are you working with on the Graph-PoC project?
Manuel Bäuscher: For the Graph-PoC project, we are working with Professor Vera Meyer’s Department of Microbiology. And at the beginning of the project in 2018, we worked with Professor Meyer, the project initiator, and Professor Lang, who applied for a patent for this sensor and its functional principle. For the detection molecule developments, the Berlin based company APTARION Biotech AG was in charge. The encapsulation of the sensor has been done by TU-Berlin Assembly and Encapsulation Technology group. Verification electronics was developed by AlphaBoard GmbH and the graphical user interface with the software control has been created by MicroDiscovery GmbH. Furthermore a clinical evaluation was planned to be done at the immunology department of Charité Berlin. A great benefit in this project was the convenience of being in the same city during the whole project duration, because all partners are Berlin based.
Can you tell us a bit about the challenges you encounter when working on the project?
Manuel Bäuscher: The electrochemical measurements are very difficult, because these molecules are very small and the measurement technology has to be sensitive enough to match them. This in turn makes it very, very difficult to determine how many capture molecules we have coupled to the surface or how many markers are in a droplet that we put on the sensor surface. That’s a lot of random variables to consider, and that makes it harder. The measurement buffer plays an important role for the stability of the sensor signal, and the biomolecule interaction do as well.
And what are the applications for these sensor platforms, apart from medical technology?
Manuel Bäuscher: They could be used not only in medical technology, but also for environmental sensor technology, which is something we are working on. We are using the combination with a metal oxide for gas detection, to detect carbon dioxide or carbon monoxide at low temperatures. It would be nice if you could build something like that into your cell phone, so that your phone could tell you when to open your window. If there is too little oxygen or too much carbon dioxide in the room or even carbon monoxide, there could be a warning system that says: Open the window quickly, or you will pass out. The advantage would be that the sensor surface doesn’t need to be heated as much as with commercial MOX gas sensors.
What are your plans for after you finished this project in September 2021?
Manuel Bäuscher: I would like to use the results to acquire a new project. Either directly with industrial partners, who can use this sensor commercially or, again, as a research project 2.0.
This interview was conducted and edited by Jacqueline Kamp
Pictures: Fraunhofer IZM





Add comment